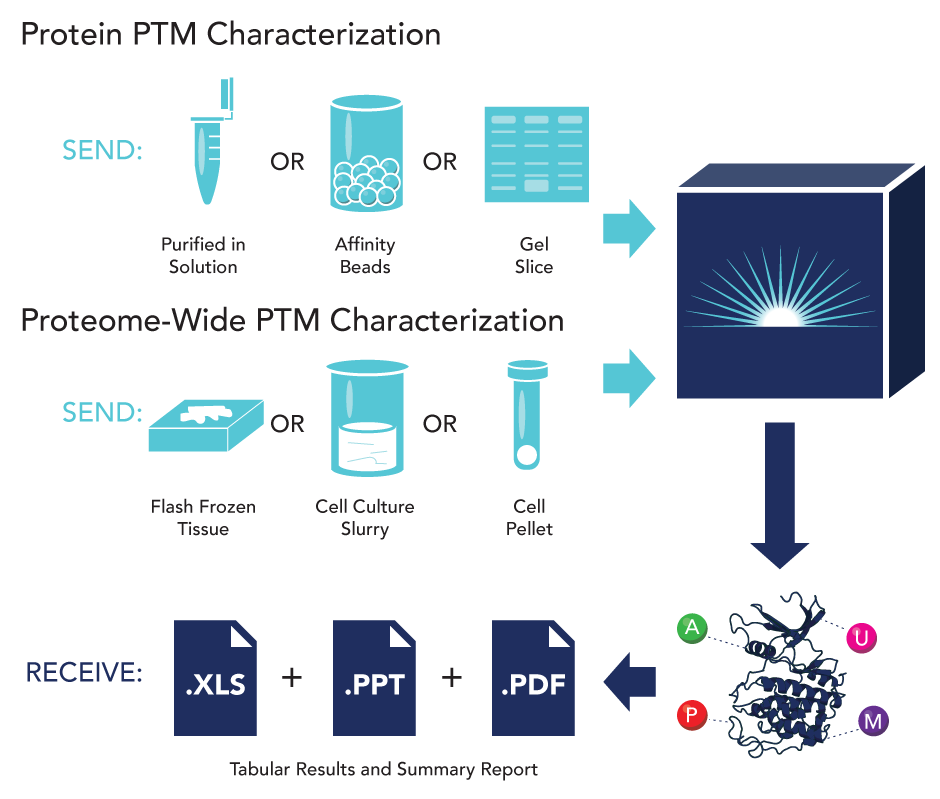
Protein post-translational modifications can modulate the range of activities and alter the interactions of proteins within a cell’s proteome. Protein PTMs allow for a variety of modified cell function including varied signaling processes, cellular differentiation, and gene expression regulation. Modifications such as phosphorylation, acetylation, methylation, and ubiquitination are added or removed from key proteins in response to disease states or drug treatment conditions. A critical step in understanding these dynamic changes within the proteome is to establish a routine method to access and identify the wide variety of PTMs that are present in the cell.
Detection of PTMs is challenging due to their low frequency and relative abundance, making identification protein PTMs from native samples very difficult. However, utilizing optimized immuno-affinity capture methods coupled with the high sensitivity and specificity of current LC-MS/MS technology, PTM characterization of complex samples can be routinely performed.
© 2015 Lighthouse Proteomics LLC. All Rights Reserved.
Designed by Peak Design Firm